Patient Engagement Platform for Healthcare Organizations
250K+ monthly engagements. 88% retention. White-label ready.
Comprehensive patient support solution with mobile app for daily health tracking and provider dashboard for remote monitoring, care coordination, and CCM/RPM billing.
What Healthcare Platforms Are Missing
Clinical software handles documentation and billing. But no mobile app means no patient data between visits.
No Patient-Facing Mobile Solution
Clinical software platforms lack consumer-grade mobile apps for daily patient engagement. Without a patient health tracking app, practices can’t capture medication adherence, symptom patterns, or lifestyle data between appointments.
Administrative Burden on Clinical Staff
Manual patient outreach, phone call follow-ups, and paper-based tracking create administrative overhead. Staff spend hours on data entry and patient check-ins that could be automated.
No Scalable Patient Engagement Tools
Existing patient portals focus on appointment scheduling, not daily health engagement. Providers lack tools to keep patients actively involved between visits, leading to poor adherence and preventable complications.
Lost Billing Revenue & Documentation Gaps
Without automated tracking, practices miss CCM/RPM billing opportunities worth $65-125 per patient monthly. Manual documentation of care coordination time is time-intensive and error-prone, leaving significant revenue uncaptured.
Proven Use Cases Across Specialties
Healthcare organizations deploy CareClinic across multiple specialties and care models to improve outcomes, increase reimbursement, and reduce costs.
Chronic Care Management (CCM)
Design and manage care plans remotely for patients with chronic conditions. Monitor patient data, coordinate care globally, and automatically document non-face-to-face time for CMS reimbursement.
Patient Reported Outcomes (ePROs)
Collect validated patient-reported outcome measures including PHQ-9, GAD-7, PROMIS, pain scales, and custom assessments.
Pre & Post-Operative Care
Track surgical readiness with pre-op checklists, monitor daily recovery progress with wound care photos, pain assessments, and activity logs.
Preventative Health Programs
Engage populations with preventative care pathways, risk stratification, adherence support, and outcomes tracking for employers, payers, and health systems.
Proven Mobile App + Provider Dashboard
250K+ monthly engagements. Validated clinical scales (PHQ-9, GAD-7, PROMIS). White-label deployment ready.
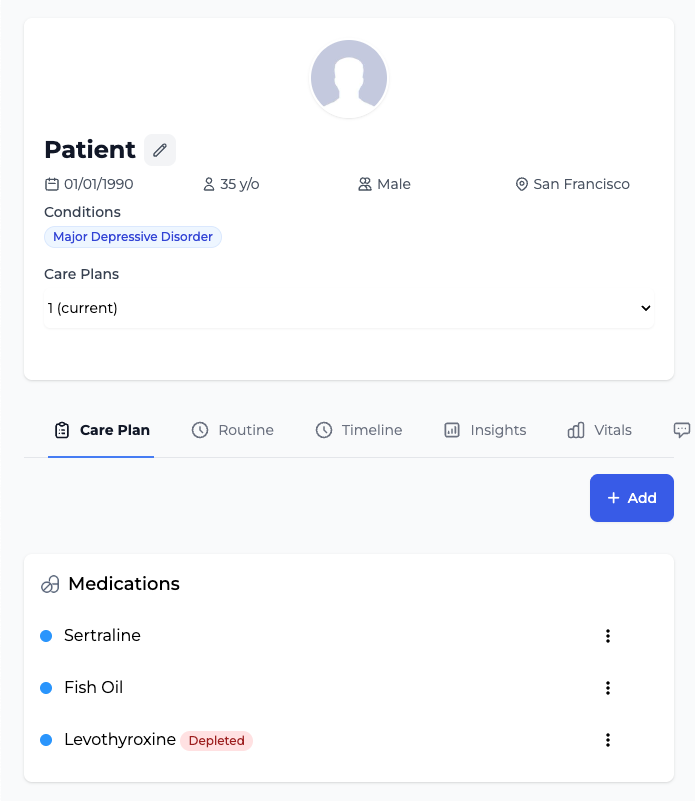
Medical-Grade Patient Data Capture
Comprehensive patient health tracking with validated assessment scales (PHQ-9, GAD-7, PROMIS) and structured data collection. Provider dashboard delivers clinical-grade insights with data export capabilities.
Real-Time Provider Alerts & Monitoring
Instant notifications for critical values, missed medications, or concerning symptom patterns. Configurable alert thresholds ensure providers intervene before complications arise, reducing ER visits and hospital readmissions.
AI-Ready Clinical Intelligence
Rich longitudinal patient data enables predictive analytics, automated risk stratification, and clinical decision support tools that enhance your platform’s capabilities.
White-Label Ready & Compliant
HIPAA-compliant infrastructure with white-label customization that allows seamless integration into your existing product suite. Deploy under your brand with full control.
Three Simple Steps
1. Platform Configuration
Set up your organization’s profile, customize branding, configure provider dashboard access. White-label deployment completed in under 2 hours.
2. Train Clinical Staff
Attend a single 1-hour virtual training session to learn the provider dashboard, patient enrollment process, and reporting tools.
3. Enroll Patients
Invite patients via email or during appointments. They download the app and start tracking with no complex setup required.
Frequently Asked Questions
Ready to Transform Patient Support?
See how CareClinic can help your organization improve patient outcomes, increase reimbursement, and reduce healthcare costs.
